- << LexSupplements
- Operate in Spain
Where do you operate from?
We help you notify or register your food supplements in the corresponding Spanish region.
Companies located in Spain must notify or register the market placement of food supplements in the Autonomous Community where the company's registered office is located, which must have previously been registered as a food business operator (FBO) in the Spanish Sanitary Registry.
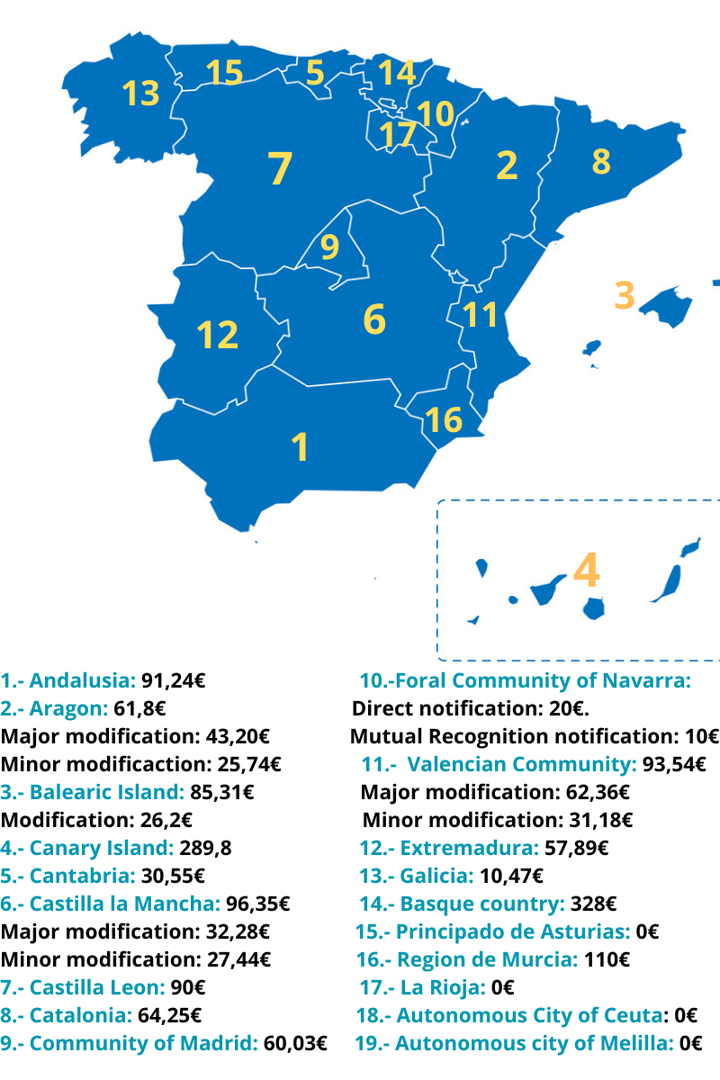
Since there is no centralization or unification of the procedure, it is necessary to adhere to the specific criteria or procedures of each Autonomous Community regarding the notification or pre-market authorisation of food supplements, as well as any potential notification fees they may impose. Thus, there are Communities that do not impose a notification fee for the food supplements and others that do. Moreover, the fee amount is variable between the different regions and some of them even set different fees depending on whether it is the initial registration, a significant modification notification, or a minor modification notification of food supplements.
Additionally, besides considering the notification procedure according to the Autonomous Community, we must determine whether the notification of the food supplement should be done directly in Spain or by using the Mutual Recognition Principle. This will depend on the composition of the product, i.e., whether it consists of ingredients regulated by Spanish and harmonized European Union legislation or not.
Specifically, if the product contains ingredients regulated and covered by the food supplements legislation in Spain (Spanish Royal Decree 1487/2009 on food supplements) or is composed of ingredients regulated by harmonized EU legislation (Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on vitamins and minerals or Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 establishing the Union list of novel foods), and moreover, if the quantities in the product comply with the maximum permissible amounts for certain ingredients, the product can be notified directly in Spain.
On the contrary, if the product contains ingredients that are not regulated or do not comply with the Spanish Royal Decree on food supplements, the product can only be notified and marketed in Spain by applying the Principle of Mutual Recognition through a prior notification and marketing in another European Union Member State.
Following map shows the different notification fees applicable for the current year for the market placement notification of food supplements in Spain:
We are specialists in food supplements! We assist you with the review and notification procedures for food supplements in all the Autonomous Communities of Spain.